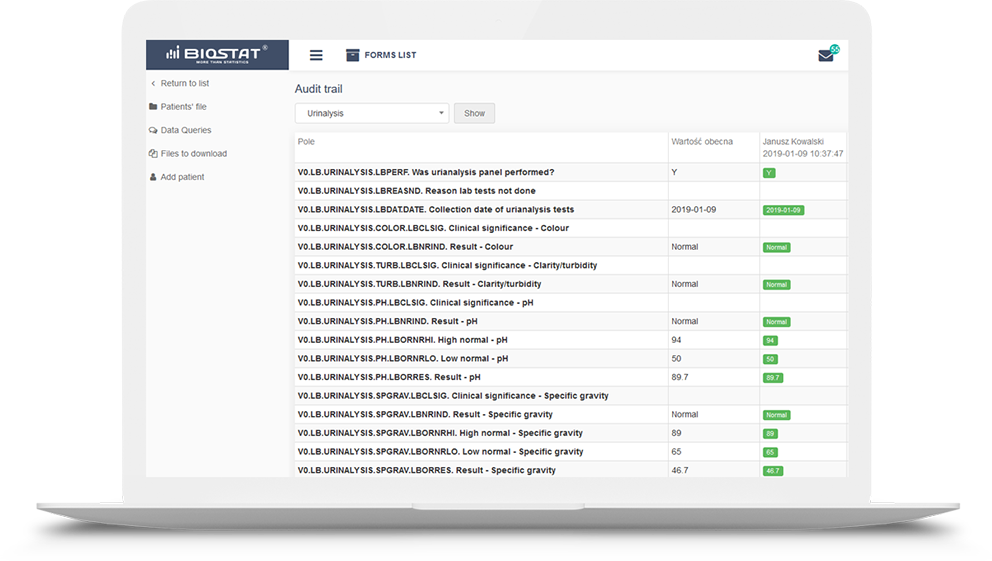
Audit trail
a fundamental function in clinical trials that every eCRF system should have. The presence of this module ensures data traceability and reliability. The audit trail module has been prepared following the
FDA (21 CFR Part 11)
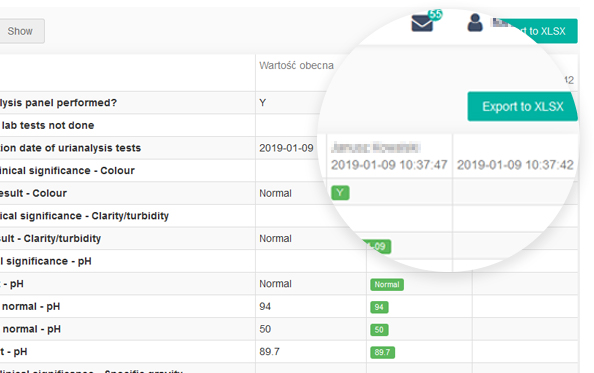
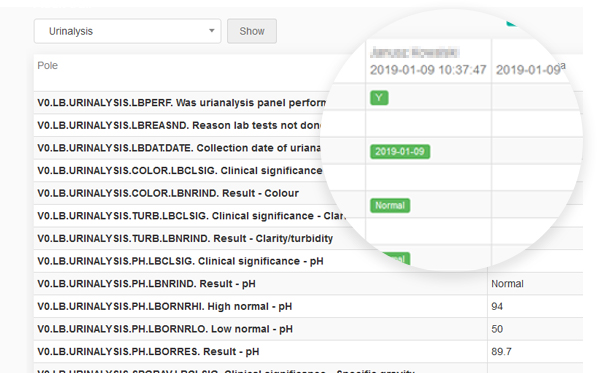
guidelines. It allows reconstruction of the history of such events relating to the record regardless of its medium, including the “who", "what", "when" and "why” of the action. This module tracks changes and activities in the system, and helps to maintain transparency and accountability.
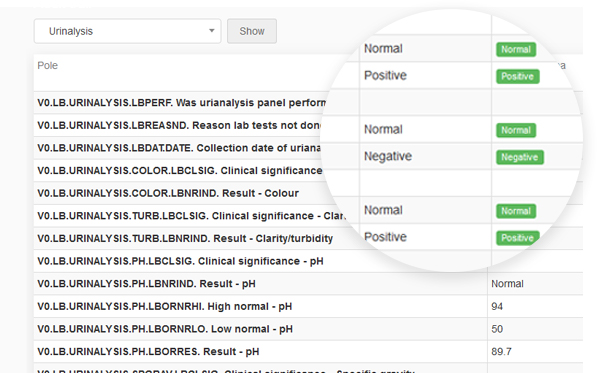
Every change in the questionnaire between two events made by each user is recorded.
Frequently asked questions.