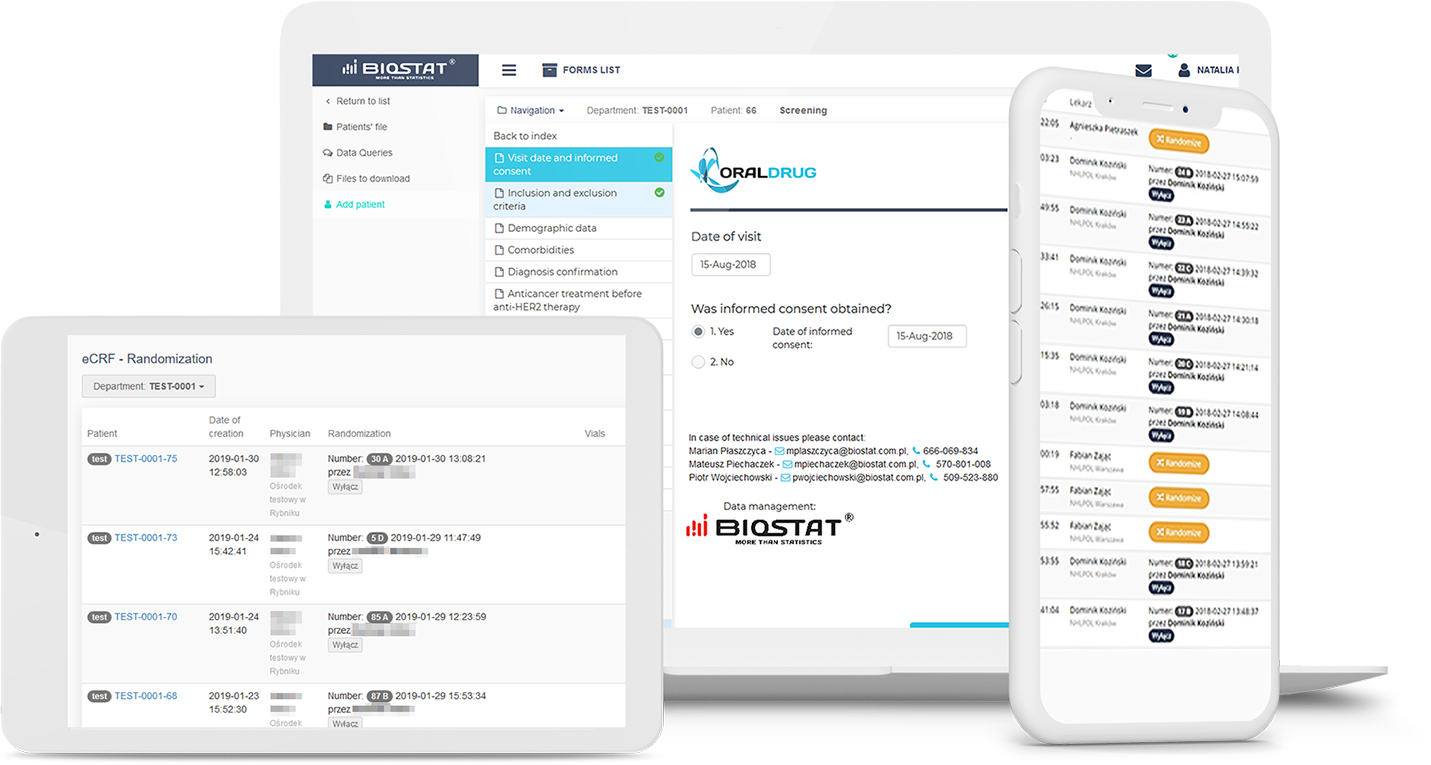
The status management of investigational medical products (IMP) allows trackinga history of blinded drug kits and display to the physicians kit numbers from the assigned study arm.
IWRS is user friendly and easy to access from anywhere in the world. In addition to the service and 24/7 availability, IWRS has many other advantages that make it attractive in clinical trials, notably large-scale studies,especially those with various treatment arms, and complex dosing schedules.



 Easy integration
Easy integration Straightforward implementation
Straightforward implementationFrequently asked questions.