
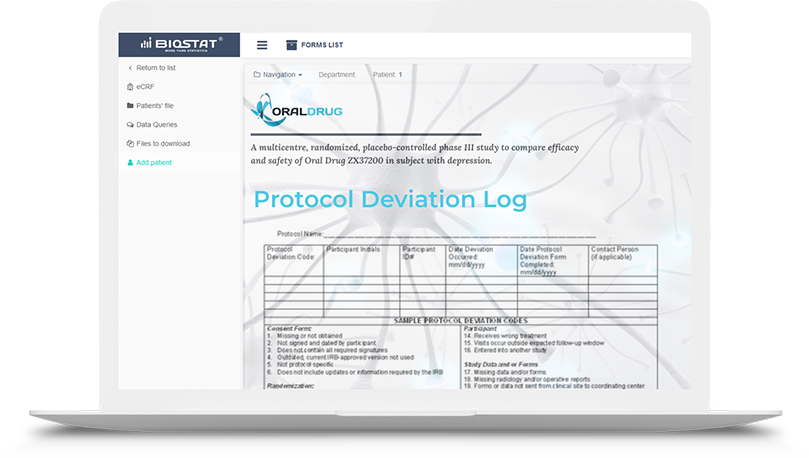
The eCRF.bizTM system contains many useful tools to facilitate monitoring of clinical trials and observational studies. One of the newest feature is protocol deviation tracker - a module that allows Monitor quick reporting of protocol deviations, precise categorization of deviations, as well as generating deviation reports directly within the eCRF.bizTM system.
Traditionally, protocol deviation log is a paper documentation or an Excel file. This new
approach s designed to automate subsequent activity throughout the entire study process.
The tool is complementary and does not replace the requirement of reporting of potential, major
protocol violations to the Sponsor or Regulatory Authority.
Frequently asked questions.