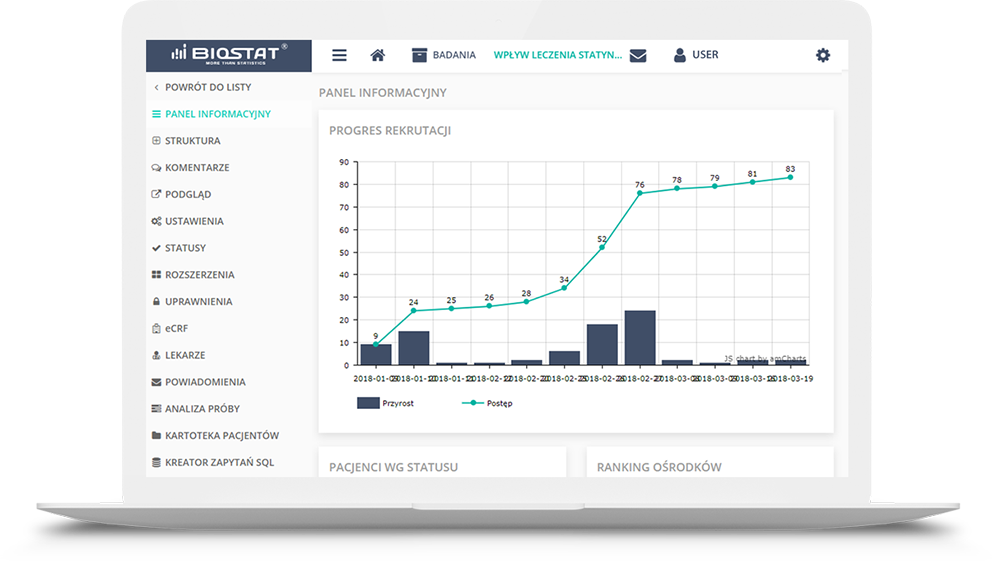
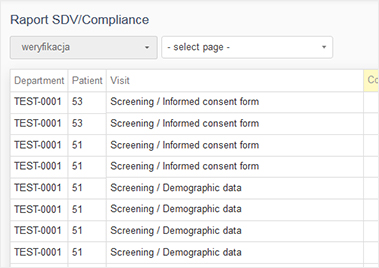
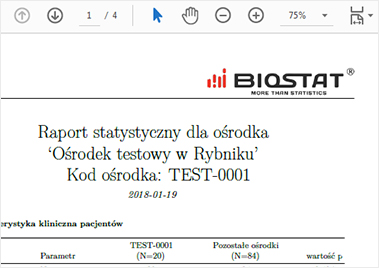
The report may be presented as a PDF or MS Excel file or a website. It may contain tables, charts and even
descriptions updated dynamically in parallel with the study progress.
These reports influence transparency of the study progress, and through the implementation of the results
comparison module between sites participating in the study, Investigators may anonymouly compare, for
instance, their therapeutic solutions with those used by their colleagues from other sites.This may be
especially useful in observational studies or registers. Another functionality is to generate a interim
analysis template that is current at a given moment without the need to involve a statisticians. This is
particularly useful tool in the early detection of abnormalities or protocol deviations, or even an
unexpected increase in the frequency of adverse events associated with the IMP.
The use of the R language allows a statistical team to be involved while freeing up eCRF programmers.
Additionally the generated code can be re-used to prepare a final statistical report.
Increase the maximum availability and legibility of information collected in the eCRF system.